The Sinnis Laboratory is part of the Johns Hopkins Malaria Research Institute, committed to the pursuit of basic science research that translates into solutions targeting one of the most important infectious diseases in the world. Malaria, caused by protozoan parasites of the genus Plasmodium, is transmitted to humans by infected Anopheles mosquitoes. The evolution of drug resistance by the parasite, and insecticide resistance by the mosquito, have created an urgent need for new strategies to control and ultimately eradicate this scourge.
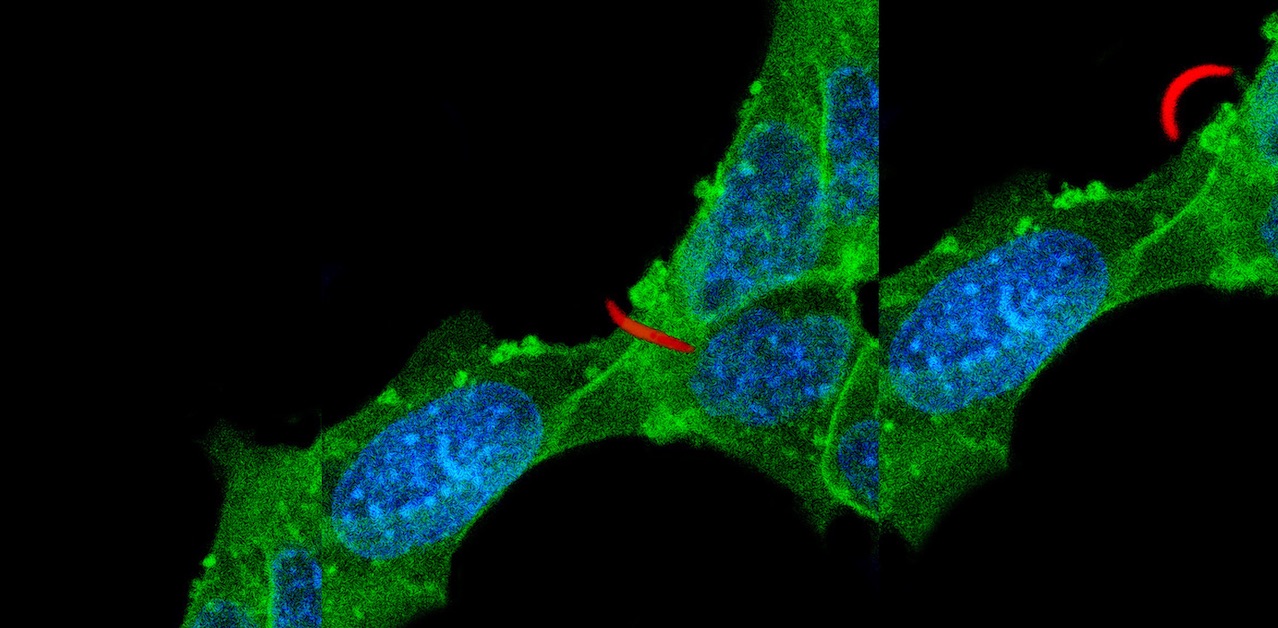
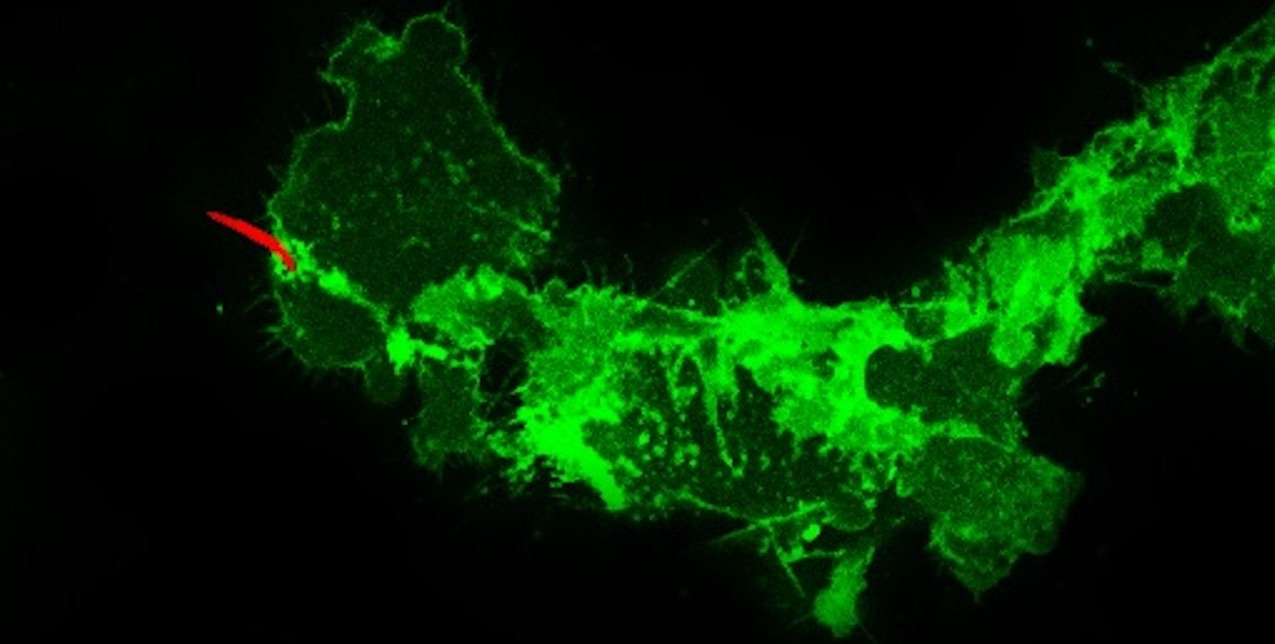
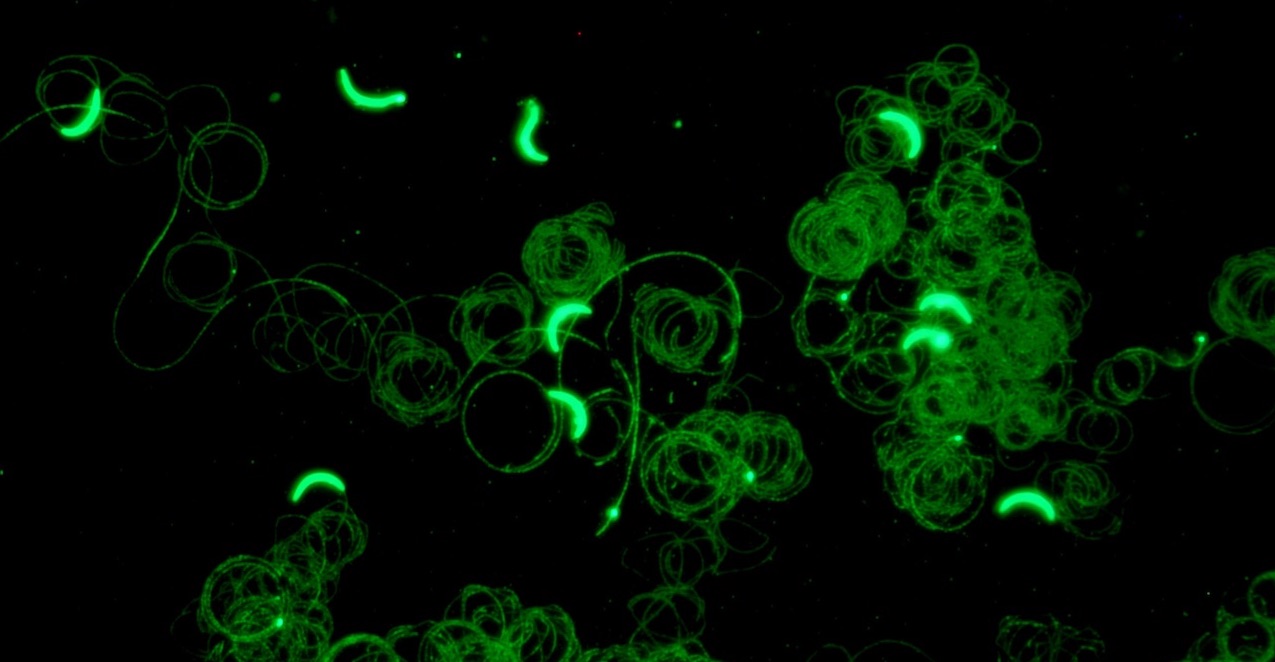
Our research focuses on the infective stage of the malaria parasite, sporozoites, which are inoculated into the skin by infected mosquitoes. Sporozoites make an impressive journey from the midgut wall of the mosquito where they emerge from oocysts, to their final destination, the mammalian liver (see picture below). Using biochemical, cell biological, and genetic approaches as well as intravital imaging and proteomics, we aim to understand the molecular interactions between sporozoites and their mosquito and mammalian hosts that enable the parasite to initiate infection. Recently we have started investigating the quantitative dynamics of sporozoite transmission, an understudied yet important area of inquiry if we are to improve our epidemiologic models and define the minimum efficacy required of transmission blocking interventions.
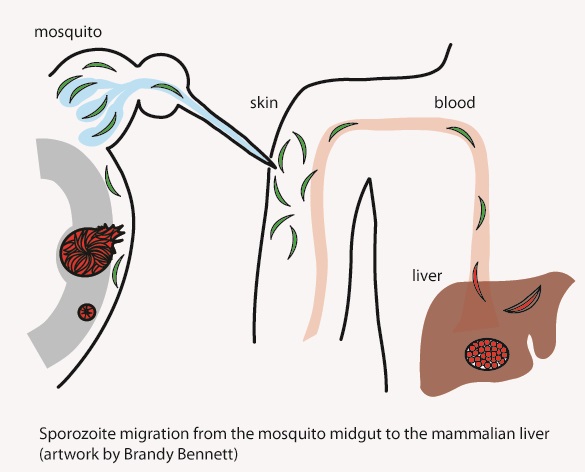
Mosquitoes inoculate sporozoites into the dermis of the mammalian host as they probe for blood. Once inoculated, sporozoites actively move through the dermis to find a blood vessel, which they penetrate to enter the circulation and go to the liver. Using a rodent malaria model we have determined the sporozoite inoculum (Medica & Sinnis 2005), the kinetics with which sporozoites exit the dermis (Yamauchi et al. 2007), and the probability of infection after a single infectious bite (Aleshnick et al. 2020); establishing that this is a bottleneck for the parasite and a promising target for intervention. More recently, we are investigating sporozoite behavior and host interactions at the inoculation site using quantitative intravital imaging (Hopp et al 2015; Hopp et al. 2021). Ongoing projects focus on the innate and adaptive immune response to sporozoites at the inoculation site, understanding the mechanism(s) by which sporozoites recognize and penetrate blood vessels, and connecting the dots between mosquito parasite burden and disease likelihood in the mammalian host.
CSP, is the major surface protein of the sporozoite, forming a dense coat on the surface of the parasite. CSP is also the basis of the only malaria vaccine to show efficacy in Phase III clinical trials, namely RTS,S. Despite its importance, our understanding of CSP structure and function is limited and we believe that elucidating how this protein functions will inform future malaria vaccine design.
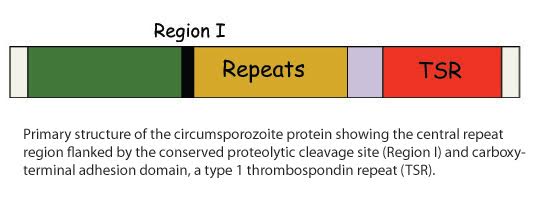
Our previous studies demonstrate that CSP has two conformational states and that this enables the sporozoite to make its remarkable journey from the mosquito midgut to the mammalian liver. During its migration phase, the cell-adhesion domain in the carboxy-terminus is masked by the N-termial domain which is then proteolytically cleaved when the parasite reaches the liver (Coppi et al. 2005; Coppi et al. 2011). Processing is triggered when sporozoites contact the highly sulfated heparan sulfate proteoglycans in the liver, which serves as a signal to the sporozoite that it has arrived in the liver and to switch from a migratory to an invasive state (Coppi et al. 2007). We are currently interested in determining the structure of the N-terminus of CSP, the function of the central repeat region and the identity of the parasite protease that cleaves CSP.
Our work on CSP and another sporozoite adhesin, TRAP (Ejigiri et al 2012), demonstrates the vital role that parasite proteases play in sporozoite motility and infectivity. We are actively working to identify the CSP protease and have expanded our work to dissect the function of other Plasmodium serine and cysteine proteases expressed by pre-erythrocytic stages. Ultimately the goal of this work is to screen inhibitors of critical proteases and validate them as drug targets.

Research Associate Scientist

Postdoctoral Fellow

Postdoctoral Fellow
Biologist,
National Institute on Aging, National Institutes of Health
Graduate Student,
Penn State University, PA
Senior Research Scientist,
Novartis Institutes for Biomedical
Research
Senior Scientist,
IDEXX
Graduate Student,
Rutgers University, NJ
Instructor,
University of Washington Center for Emerging Infectious Diseases
Undergraduate Student,
Bard College, NY
Laboratory Technician,
New York City, NY
Senior Research Associate,
Oregon Health and Science University, OR
Staff Scientist,
Naval Medical Research Center, MD
Retired,
Relaxing & spending time with family in China
Visiting Fellow,
National Institutes of Health, MD
Senior Scientist,
AstraZeneca
Senior Scientist,
CSIR-Central Drug Research Institute, India
Director of Program Management,
Regeneron Pharmaceuticals
Graduate student,
Columbia University
Senior Staff Scientist,
Regeneron Pharmaceuticals
Physician,
Jencare
Adjunct Professor
Emory University School of Medicine
Professor,
Pediatric Infectious Diseases, University of Mississippi Medical Center
Group Leader,
Malaria Regulation Laboratory, Paris-Saclay University, France
Associate Professor,
Biological Sciences Department, City University of New York
Professor,
Universidade Estadual de Londrina, Parana, Brasil
Professor,
Department of Biology, Pennsylvania State University
Program Officer,
National Institutes of Health, MD
Scientist,
AstraZeneca
June 2021: Orna Rabinovich Ernst joins the Sinnis lab from the NIH where she studied innate immune signaling! Welcome Orna!
April 2021: Sharon Patray gave a virtual tour of the lab for the Henderson Hopkins Middle School students. Watch it here!
January 2021: Christine's and Sachie's paper on sporozoite motility in the skin was chosen for the
cover of EMBO Molecular Medicine!
July 2020: Sharon Patray was awarded the Diversity Supplement from the National Institutes of Health
June 2020: Congratulations to Ritwik Sighal for completing his Master of Science! Good luck at Penn State University!
September 2018: Amanda Balaban gave a great talk at the Molecular Parasitology meeting in Woods Hole.
May 2018: Congratulations to Dr. Amanda Balaban for successfully defending her doctoral dissertation!
March 2018: Photini, together with Peter Crompton, talk about "The Challenge of Malaria: The World's Number One Killer" in the NIH's Demystifying Medicine course
October 2017: Sinnis Lab awarded Honorable Mention in the 2017 Nikon Small World in Motion Competition
September 2017: Gibbs Nasir was featured in a short video about the day in the life of a Hopkins Bloomberg Public Health Student
September 2017: Melanie Shears received a Best Poster Award at the 2017 Molecular Parasitology Meeting in Woods Hole
June 2017: Photini was elected as a Fellow of the American Society of Microbiology
Summer 2017: Photini is co-directing the Biology of Parasitism Course at the Marine Biological Laboratory in Woods Hole, Massachusetts
May 2017: Congratulations to our Graduates! Gibbs Nasir, Natasha Vartak, and Enoch Chan received their Master of Science degrees
April 2017: Sinnis lab research featured in a video about the Johns Hopkins Malaria Research Institute Click Here to Watch!
January 2017: Melanie Shears received a Johns Hopkins University Provost's Postdoctoral Fellowship and was recognized at the Annual Honors and Awards Ceremony. Congratulations Melanie!


















Gliding Motility Trail Assay
Infecting Mosquitoes With Rodent Malaria Parasites
Mosquito Salivary Gland Dissections
Sporozoite Isolation & Counting
Johns Hopkins Malaria Research Institute
Dept. Molecular Microbiology & Immunology PhD Program
Dept. Biochemistry & Molecular Biology PhD Program
Cellular & Molecular Medicine PhD Program
Malaria Institute Insectary Core Facility
Malaria Institute Parasite Core Facility
PlasmoDB: The Plasmodium Genome Resource
MVI PATH Malaria Vaccine Initiative
Internal MMI Portal
Johns Hopkins iLabs
Sinnis Lab Equipment Calendar
Johns Hopkins Bloomberg School of Public Health
Dept. Molecular Microbiology & Immunology
615 N. Wolfe St.
Baltimore, Maryland 21205,
United States
© 2020 The Sinnis Laboratory. All rights reserved